Resistivity of Gold
An educational, fair use website
| Bibliographic Entry | Result (w/surrounding text) |
Standardized Result |
|---|---|---|
| Cutnell, John D & Kenneth W Johnson. Physics. 4th Edition. New York: Wiley, 1998: 591. | "Table 20.1 Resistivities of Various Metals Material Resistivity (ohm-m) Gold 2.44 × 10−8" |
2.44 × 10−8 Ω·m |
| Lloyd, David H. Physics Laboratory Manual. 2nd Edition. Florida: Saunders college Publishing, 1998: 562. | "Table II D Resistivities and Temperature Coefficients Substance Resistivity (ohm-m) Temperature Coefficient (°C)−1 Gold 2.44 × 10−8 3.4 × 10−3" |
2.44 × 10−8 Ω·m |
| Properties of Gold. World. Gold Council, May 2004. | "The electrical resistivity of gold is 0.022 micro-ohm m at 20 °C" | 2.20 × 10−8 Ω·m |
| Seitz, Frederick. The Modern Theory of Solids. New York: Dover Publications, 1987: 10. | "Table III- The Resistivities of Metals at Room Temperature (The resistivity ρ is expressed as 10−6 ohm-cm. ∥ and ⊥ designate, respectively, values in directions parallel and perpendicular to the principal axis in hexagonal and tetragonal crystals.) Monovalent Metals Au 2.04" |
2.04 × 10−8 Ω·m |
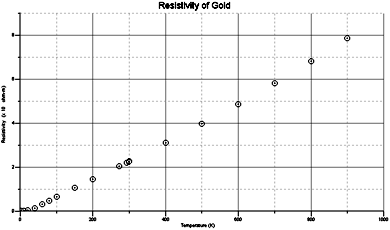
| Lide, David R. Handbook of Chemistry and Physics. 75th Edition. New York: CRC Press, 1997: 11-41. | [see table and graph below] | [see below] |
"Gold is the child of Zeus, neither moth nor rust can devoureth it." -- Pinder
Beyond the realm of glitz, glamour and pomp, gold (Au) stands in the company of metals like silver, copper, and aluminum as one of the best conductors known to man. Though its beauty is unsurpassed, gold is much more than mere candy. Throughout the ages it has offered the world a myriad of uses, ranging from coinage and the age-old allure of jewelry to electronics and (gasp!) even dentistry.
Nowadays in an age where technology reigns and rules, gold's many valuable physical and chemical properties are put to use each and every day. As the most non-reactive of all metals, gold is unaltered by factors like heat and moisture, and never reacts with oxygen, meaning it will never degrade, rust or tarnish over time; it is this property that renders gold an ideal metal. Gold's unparalleled ductility and malleability has made it a craftsman's dream; it can be drawn into wires miles and miles long, and can be easily shaped. And above all, gold is an excellent conductor of heat and electric current, a quality that has made it essential in the field of electronics. With a plethora of virtues like an extremely high resistance to corrosion and an ability to conduct both heat and electric current, gold serves as the single-most reliable and indispensable metal in this field. Whether it be a computer, a television set, radio, cellular phone, or even your hum-drum, everyday washing machine, don't be too surprised to find that gold performs the most important functions in such devices and a host of others. Alas you can't quite plan your next get-rich-quick scheme based solely on this fact. In (harsh) reality, very thin layers of gold are used to electroplate contact points in electrical devices. Modems, printers, cameras, fax machines, and even compact discs all rely on gold to function properly. For example, telephones use gold-coated contacts to provide clear reception, gold is used in the crash sensors of automobile air bags, and year after year gold is used in the microchips and circuit boards of millions of computers to ensure reliable signals. Nonetheless, the usefulness of gold reaches far and beyond the world of electronics; gold also plays vital roles in medicine, architecture, dentistry, the arts, and even in aerospace.
As one of the most sought-after metals in the world, gold has a wide range of valuable chemical and physical properties. Not the least of which is a property called resistivity. While the electrical resistance of a substance's opposition to the flow of charge, resistivity measures how well a substance resists carrying a current, and is dependent on the chemical composition of the substance. Good conductors have low resistivities while bad conductors and insulators have high resistivities; hence resistivity is also known as the reciprocal of conductivity. As the graph shows, the resistivity of a substance is very much dependent on temperature. For most conductors, resistivity increases with rising temperature. At 20 °C, the resistivity of gold is approximately 2.44 × 10−8 ohm-m and steadily rises with increasing temperature. The temperature coefficient of a substance measures the amount of increase in the resistance of a 1 ohm sample of the conductor per degree rise in temperature (in Celsius). A positive coefficient indicates that a material's resistance increases with a rise in temperature, and vice versa. Pure metals, like gold, usually have positive temperature coefficients.
Jennelle Baptiste -- 2004
External links to this page:
- US Patent Application 12/900,976. Self healing high energy glass capacitors. Michael Lanagan, et al. 2010.
- Features of Radiation Heating Kinetics of Metal Plates under Fluctuation-Electromagnetic Friction. G.V. Dedkov. Journal of Experimental and Theoretical Physics (JETP) Letters. Vol. 121 (2025): 154–158.
- Сила трения Казимира-Лифшица и кинетика радиационного теплообмена металлических пластин при относительном движении. Дедков Г.В. Письма в Журнале Экспериментальной и Теоретической Физики (ЖЭТФ). том 117 вып. 12 (2023): 950–955.