Density of Water
An educational, fair use website
| Bibliographic Entry | Result (w/surrounding text) |
Standardized Result |
|---|---|---|
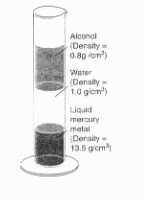
| McGuire, Thomas. Earth Science: The Physical Setting. New York, AMSCO, 1998: 123. | [see image 1 below] | 1 g/cm3 |
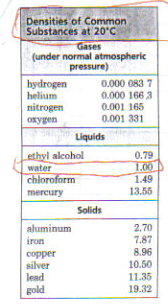
| Cohen, Paul. Geffer, Saul. Chemistry A Contemporary Approach. New York. AMSCO, 1994: 18. | [see image 2 below] | 1 g/cm3 |
| Dorn, Henry. Chemistry: The Study of Matter: Fourth Edition. Englewood Cliffs, NJ: Prentice Hall 1987: 72. | [see image 3 below] | 1 g/cm3 |
| Density of Water, Simetric. October 9, 2004. | [see table and graph 5 below] | 1 g/cm3 |
Water is a very common substance here on Earth. Almost 75% of the Earth's surface is covered with water and almost every living thing on Earth is made up of 90% water. Water can change into three phases of matter. Water is most common in it's liquid state when it is kept a normal pressure and between 0 degree Celsius and 100 degree Celsius. Water turns to ice as it's solid state from 0 degrees Celsius and below. Water turns into steam from 100 degrees and above.
Density is defined as mass per unit of volume. The commonly used formula to determine the density of an object is ρ = m/V, ρ (rho) represents density, m represents mass, and V represents volume. The units used to indicate density are [kg/m3] or more commonly used [g/cm3]. The conversion between the two is 1000 kg/m3 to 1 g/cm3.
Water never has an absolute density because its density varies with temperature. Water has its maximum density of 1g/cm3 at 4 degrees Celsius. When the temperature changes from either greater or less than 4 degrees, the density will become less then 1 g/cm3. Water has the maximum density of 1 g/cm3 only when it is pure water. Other factors affect water's density such as whether it is tap or fresh water or salt water. These variations of water changes its density because what's in the water has its own density.
 |
 |
 |
|
 |
| Temp ( °C) |
Density pure water (g/cm3) |
Density pure water (kg/m3) |
Density tap water (g/cm3) |
Density pure water lb/cu.ft |
Specific Gravity 4 °C reference |
Specific Gravity 60 °F reference |
|---|---|---|---|---|---|---|
| 0 (solid) | 0.9150 | 915.0 | – | – | 0.915 | – |
| 0 (liquid) | 0.9999 | 999.9 | 0.99987 | 62.42 | 0.999 | 1.002 |
| 4 | 1.0000 | 1000 | 0.99999 | 62.42 | 1.000 | 1.001 |
| 20 | 0.9982 | 998.2 | 0.99823 | 62.28 | 0.998 | 0.999 |
| 40 | 0.9922 | 992.2 | 0.99225 | 61.92 | 0.992 | 0.993 |
| 60 | 0.9832 | 983.2 | 0.98389 | 61.39 | 0.983 | 0.985 |
| 80 | 0.9718 | 971.8 | 0.97487 | 60.65 | 0.972 | 0.973 |
| 100 (gas) | 0.0006 | see steam tables... | – | – | ||

Graph 5
Allen Ma -- 2007